Biomedical Research
Dr. Paul Fedak is a globally recognized cardiac surgeon, scientist, and health system leader with a career of innovation, impact, and academic excellence. He serves as Professor and former Head of Cardiac Sciences at the University of Calgary. He is Director of the Libin Cardiovascular Institute, where he leads integrated initiatives in precision cardiovascular health, translational research, and system transformation.
For 25 years, I have actively participated in biomedical research and been productive in peer-reviewed original contributions. As an academic cardiac surgeon and scientist, I have explored the basic and clinical science of bicuspid aortic valve aortopathy and cardiac fibrosis and remodelling while actively treating patients with these diseases. I have also played an essential role in the translational application of biomaterials and devices in cardiac surgery.
Recently, I have positioned my research program to adopt a pioneering role in cardio-immunology related to cardiac repair mechanisms. This emerging area has significant clinical implications for cardiac surgery patients. I aim to provide clinically relevant scientific leadership and translational expertise in exploring the molecular pathways that drive immune-mediated cardiac surgical outcomes, such as post-surgical adhesions and postoperative atrial fibrillation.
Forced to retire from an active cardiac surgery practice due to cervical degeneration, I lead an active translational research laboratory with three graduate students, three technicians, and numerous collaborators. My formal scientific training (PhD, University of Toronto) was focused on extracellular matrix biology and its influence on cardiovascular tissue remodelling. I have leveraged this expertise to examine matrix remodelling in cardiac fibrosis and have made a substantial scientific contribution with numerous highly cited/high-impact publications.
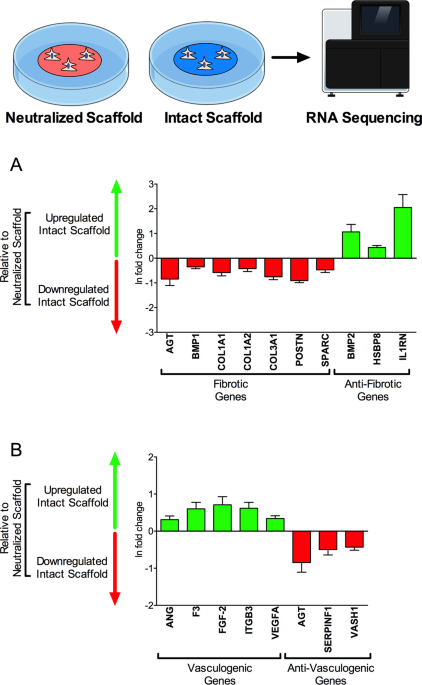
The overarching aim of my research program is to 1) define critical molecular and cellular mechanisms of ECM dysregulation in cardiovascular tissues and, 2) understand the resultant influence of ECM dysregulation on maladaptive tissue remodeling and organ dysfunction, and 3) leverage these insights into both novel clinical therapies and risk prediction biomarkers. We have made substantial insights into the role of TIMP-3 and other ECM regulatory molecules in myocardial remodelling, human myofibroblast activation, and cell therapy/tissue engineering with ECM-based biomaterials.

With expertise in ECM dysregulation in the setting of myocardial infarction, I have established essential collaborations with experts in Immunology to study the role of humoral mediators in post-myocardial injury processes. In 2019, I was the co-senior author of an original paper (Immunity 2019) that, for the first time, demonstrated the reparative properties of pericardial macrophages in an ischemic rodent model. Pioneering work from this manuscript has provided novel insights into the emerging field of cardio immunology. It has also set the foundation for current work in my lab that focuses on 1) studying the impact of pericardial immune factors on cardiac surgery outcomes, 2) investigating the interplay between biomaterials and inflammatory pathways, and 3) determining how such an association can be leveraged to improve outcomes for patients with myocardial infarction and those undergoing cardiac surgery.
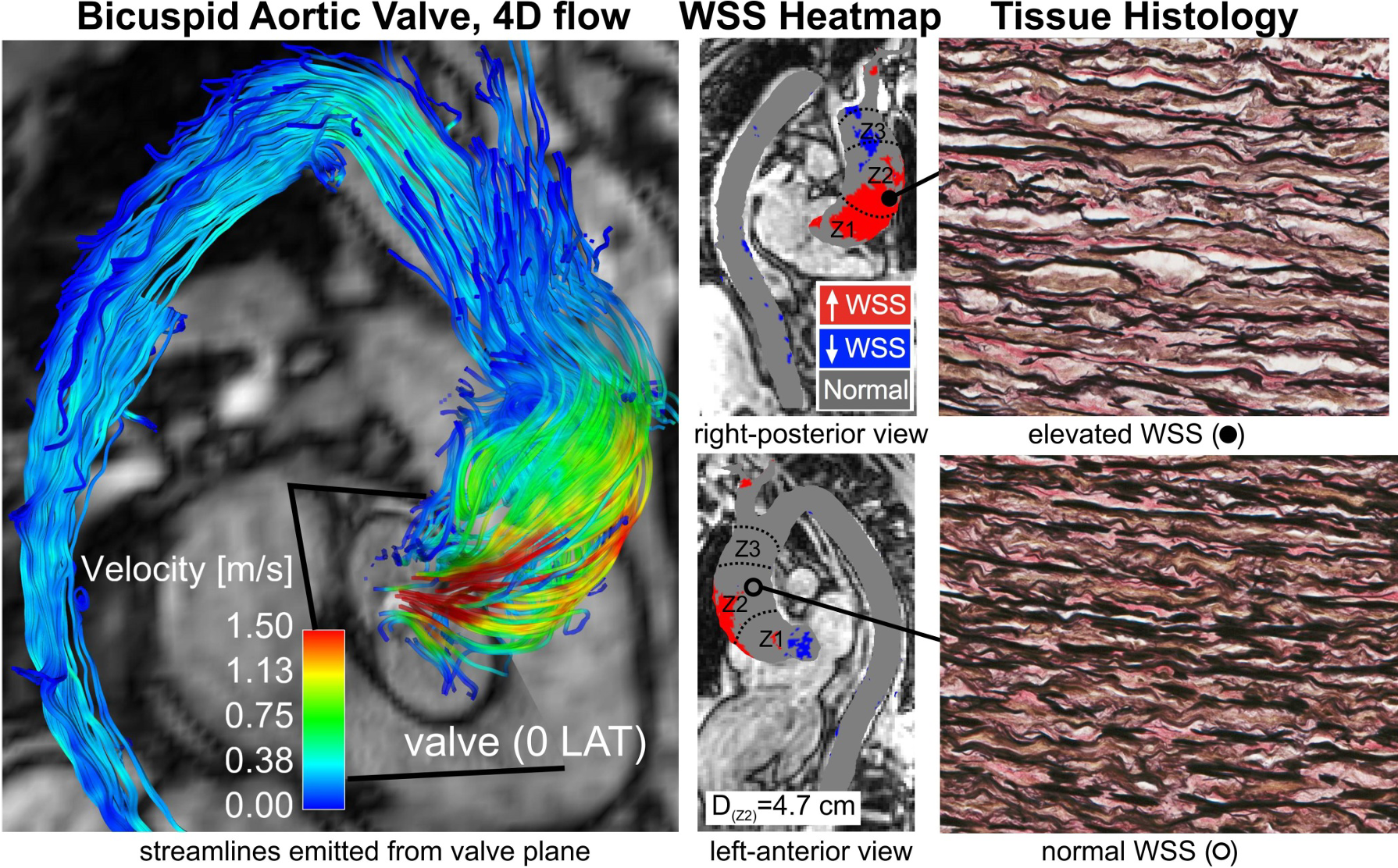
My collaboration with Northwestern University has evolved since my clinical fellowship training in Chicago in 2006. We have made impactful original contributions using 4D-flow MRI to better understand the heterogeneity of BAV aortopathy and the role of valve-mediated hemodynamics. We discovered that a bicuspid aortic valve (without clinically significant dysfunction) creates abnormal downstream vessel wall forces, signifying that the valve function alone (stenosis or regurgitation) is inadequate to define the risk of aortopathy. These studies support the potential of 4D-flow MRI to define maladaptive valve-mediated hemodynamics in individual patients with BAV, and provide a proof-of-concept that observed hemodynamic stresses can influence local tissue remodeling and disease progression.
With expertise in ECM dysregulation, I have made contributions to the understanding of molecular mechanisms of BAV aortopathy. In 2002, I published an original contribution that introduced the concept of ECM dysregulation in BAV aortopathy and discovered MMP-2 as a critical biomarker of the disease (JTCVS 2002 - 472 citations). We further explored tissue heterogeneity in human tissue samples and offered editorial perspectives on molecular biomarkers and risk prediction based on our scientific insights.
I have explored knowledge gaps in clinical risk stratification and surgical treatments for patients with BAV. We determined resection size thresholds with the risk of aortic events (JTCVS 2004 – 399 citations) that strongly influenced subsequent clinical practice guidelines (4.5 cm resection threshold). These studies have outlined the strong relationship between basic science molecular understanding of BAV aortopathy (genetics vs hemodynamics) and heterogeneity in clinical practice patterns with a clear need for improved risk prediction in individual patients with BAV.






Some Selected Links to My Publications
https://www.jacc.org/digital-content/podcast/4d-flow-mri-and-bav-aortopathy
https://www.jacc.org/doi/abs/10.1016/j.jacbts.2023.01.012
https://www.ahajournals.org/doi/10.1161/01.cir.0000027905.26586.e8
https://www.jacc.org/doi/10.1016/j.jacbts.2024.06.007
https://www.jacc.org/doi/10.1016/j.jacc.2015.06.1310